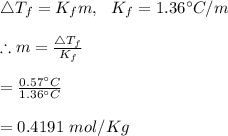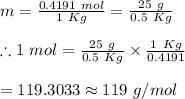Chemistry, 22.04.2020 01:46 slowik9467
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
0.57 °C what is the molecular weight of the compound assume no molecular disassociation upon
dissolution Kf equals 1.36 °C/m
O 119 g/mol
90 g/mol
0 60 g/mol
238 g/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
You know the right answer?
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
Questions


Mathematics, 13.01.2021 18:50


French, 13.01.2021 18:50


Mathematics, 13.01.2021 18:50

Mathematics, 13.01.2021 18:50

Chemistry, 13.01.2021 18:50


Mathematics, 13.01.2021 18:50

Mathematics, 13.01.2021 18:50

Mathematics, 13.01.2021 18:50


Mathematics, 13.01.2021 18:50