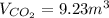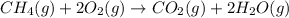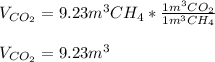Methane gas and oxygen gas react to form water vapor and carbon dioxide gas. What volume of carbon dioxide would be produced by this reaction if 9.23 m o methane were consumed? Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Methane gas and oxygen gas react to form water vapor and carbon dioxide gas. What volume of carbon d...
Questions

Mathematics, 12.10.2021 08:20

English, 12.10.2021 08:20

Mathematics, 12.10.2021 08:20


Law, 12.10.2021 08:20

Mathematics, 12.10.2021 08:20


Mathematics, 12.10.2021 08:20

Mathematics, 12.10.2021 08:20

Biology, 12.10.2021 08:20



Biology, 12.10.2021 08:20

Computers and Technology, 12.10.2021 08:20

Social Studies, 12.10.2021 08:20



Mathematics, 12.10.2021 08:20

Spanish, 12.10.2021 08:20

Biology, 12.10.2021 08:20