
Chemistry, 22.04.2020 02:08 alexis9263
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b) the entropy of the solution must be greater than that of its pure components. c) H bonds must exist between solvent and solute. d) the entropy of the solution is immaterial. e) strong ion-dipole forces must exist in the solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1


Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b)...
Questions




Biology, 13.07.2019 22:40


History, 13.07.2019 22:40

English, 13.07.2019 22:40

Mathematics, 13.07.2019 22:40


Health, 13.07.2019 22:40

Mathematics, 13.07.2019 22:40

History, 13.07.2019 22:40


Chemistry, 13.07.2019 22:40

Biology, 13.07.2019 22:40





Biology, 13.07.2019 22:40

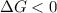 .
.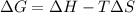 .......... (1)
.......... (1)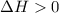 (for an endothermic process).
(for an endothermic process).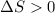 ) more will be the negative value of
) more will be the negative value of 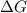 .
.

