
Chemistry, 22.04.2020 02:12 trinati6965
Calculate the DH°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide. DH°f means delta or change of heat of formation DH°f [CaCO3(s)] = –1206.9 kJ/mol; DH°f [CaO(s)] = –635.1 kJ/mol; DH°f [CO2(g)] = –393.5 kJ/mol CaCO3(s) --> CaO(s) + CO2(g)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3


Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
Calculate the DH°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide....
Questions

History, 18.07.2019 11:30

Biology, 18.07.2019 11:30



Physics, 18.07.2019 11:30


Mathematics, 18.07.2019 11:30

Biology, 18.07.2019 11:30


Biology, 18.07.2019 11:30

Business, 18.07.2019 11:30

Mathematics, 18.07.2019 11:30

Mathematics, 18.07.2019 11:30

Mathematics, 18.07.2019 11:30


Mathematics, 18.07.2019 11:30


Social Studies, 18.07.2019 11:30


Business, 18.07.2019 11:30


![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(CaO(s))})+(1\times \Delta H^0f_{CO_2}]-[(1\times \Delta H^o_f_{(CaCO_3(s))})]](/tpl/images/0617/0361/6353c.png)
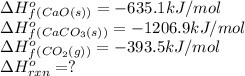
![\Delta H^o_{rxn}=[(1\times (-635.1))+(1\times (-393.5))]-[(1\times (-1206.9))]](/tpl/images/0617/0361/be80a.png)


