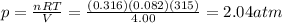Chemistry, 22.04.2020 02:18 babydoll1981
A 0.316\,\text{mol}0.316mol0, point, 316, start text, m, o, l, end text sample of nitrogen gas, \text{N}_2(g)N
2
(g)start text, N, end text, start subscript, 2, end subscript, left parenthesis, g, right parenthesis, is placed in a 4.00\,\text L4.00L4, point, 00, start text, L, end text container at 315\,\text K315K315, start text, K, end text.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
A 0.316\,\text{mol}0.316mol0, point, 316, start text, m, o, l, end text sample of nitrogen gas, \tex...
Questions

Biology, 17.07.2019 06:00


Advanced Placement (AP), 17.07.2019 06:00

Health, 17.07.2019 06:00

History, 17.07.2019 06:00


History, 17.07.2019 06:00


Mathematics, 17.07.2019 06:00



Mathematics, 17.07.2019 06:00

History, 17.07.2019 06:00






Physics, 17.07.2019 06:00

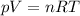
 (gas constant)
(gas constant)