Chemistry, 22.04.2020 02:39 LtotheJ0225
Nitric oxide, an important pollutant in air, is formed from the elements nitrogen and oxygen at high temperatures, such as those obtained when gasoline burns in an automobile engine. At 2000°C, K for the reaction N2(g) + O2(g) 2NO(g) is 0.01. Predict the direction in which the system will move to reach equilibrium at 2000°C if 0.4 moles of N 2, 0.1 moles of O 2, and 0.08 moles of NO are placed in a 1.0-liter container.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
Nitric oxide, an important pollutant in air, is formed from the elements nitrogen and oxygen at high...
Questions


Biology, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

Advanced Placement (AP), 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

History, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50


Mathematics, 27.03.2021 21:50

English, 27.03.2021 21:50

English, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50

Mathematics, 27.03.2021 21:50


Mathematics, 27.03.2021 21:50

Computers and Technology, 27.03.2021 21:50

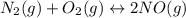
![Q=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0617/1727/a703d.png)