Chemistry, 22.04.2020 03:07 ccarman1432
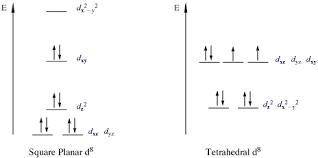
The complex ion NidppeCl2 can either have a tetrahedral geometry or a square planar geometry around the interior Ni2 ion. When tested, NidppeCl2 proved to be diamagnetic. Based on crystal field theory, what is the geometry around the central Ni ion

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

You know the right answer?
The complex ion NidppeCl2 can either have a tetrahedral geometry or a square planar geometry around...
Questions

Computers and Technology, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00


Mathematics, 02.03.2021 23:00

Chemistry, 02.03.2021 23:00



Geography, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00



Mathematics, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00



English, 02.03.2021 23:00

Social Studies, 02.03.2021 23:00