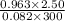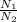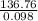You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 [infinity]C. The He is used to fill balloons to a volume of 2.50 L at 732 mm Hg and 27 [infinity]C. How many balloons can be filled with He? Assume that the cylinder can provide He until its internal pressure reaches 1.00 atm (i. e., there are 131 atmospheres of usable He in the cylinder).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 [infinity]C. T...
Questions

Mathematics, 21.04.2020 21:30




Biology, 21.04.2020 21:31

Social Studies, 21.04.2020 21:31

Mathematics, 21.04.2020 21:31





Mathematics, 21.04.2020 21:31

Mathematics, 21.04.2020 21:31

Biology, 21.04.2020 21:31



Physics, 21.04.2020 21:31



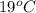 = (19 + 273) K
= (19 + 273) K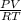
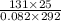
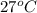 = (27 + 273) K
= (27 + 273) K