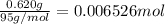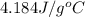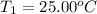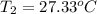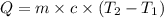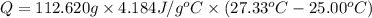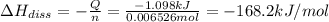Chemistry, 22.04.2020 03:29 milkshakegrande101
The salt magnesium chloride is soluble in water. When 0.620 g MgCl2 is dissolved in 112.00 g water, the temperature of the solution increases from 25.00 °C to 27.33 °C. Based on this observation, calculate the dissolution enthalpy, ΔdissH, of MgCl2. Assume that the specific heat capacity of the solution is 4.184 J g-1 °C-1 and that the energy transfer to the calorimeter is negligible. ΔdissH = kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3
You know the right answer?
The salt magnesium chloride is soluble in water. When 0.620 g MgCl2 is dissolved in 112.00 g water,...
Questions

Mathematics, 24.11.2020 01:00

Advanced Placement (AP), 24.11.2020 01:00



Mathematics, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00

English, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00



English, 24.11.2020 01:00

Biology, 24.11.2020 01:00