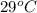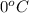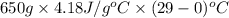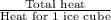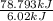Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume that each ice cube contains 1 mole of H2O and is initially at 0°C. ∆H(fusion) = 6.02 kJ/mol; ∆H(vaporization) = 40.7 kJ/mol c(solid) = 2.09 J/g°C; c(liquid) = 4.18 J/g°C; c(gas) = 1.97 J/g°C Enter your answer numerically.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

You know the right answer?
Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume...
Questions


Mathematics, 29.06.2019 13:30




Mathematics, 29.06.2019 13:30

Health, 29.06.2019 13:30

Mathematics, 29.06.2019 13:30



Mathematics, 29.06.2019 13:30

Mathematics, 29.06.2019 13:30

History, 29.06.2019 13:30




History, 29.06.2019 13:30

Mathematics, 29.06.2019 13:30


Health, 29.06.2019 13:30

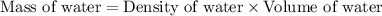
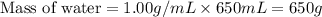
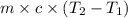
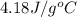
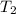 = final temperature =
= final temperature =