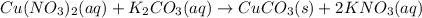Chemistry, 22.04.2020 03:35 teneciastclair7
Use the equation editor or "Insert Chemistry - WIRIS editor" to write the balanced molecular chemical equation for the reaction of aqueous 0.34 M copper (II) nitrate, with 0.70 M potassium carbonate. You may need to consult Appendix E to determine the states of each reactant and product. Assume any insoluble products are completely insoluble.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
Use the equation editor or "Insert Chemistry - WIRIS editor" to write the balanced molecular chemica...
Questions





English, 27.11.2019 01:31



Social Studies, 27.11.2019 01:31

English, 27.11.2019 01:31


Computers and Technology, 27.11.2019 01:31

Mathematics, 27.11.2019 01:31

English, 27.11.2019 01:31

Mathematics, 27.11.2019 01:31


Mathematics, 27.11.2019 01:31

History, 27.11.2019 01:31

Physics, 27.11.2019 01:31


Physics, 27.11.2019 01:31