Chemistry, 22.04.2020 03:43 eboniwiley
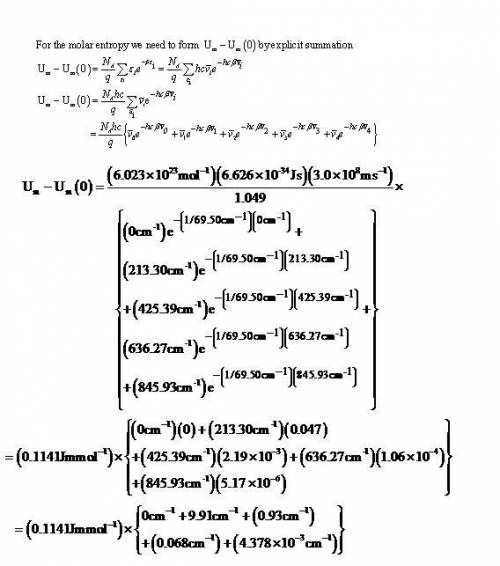
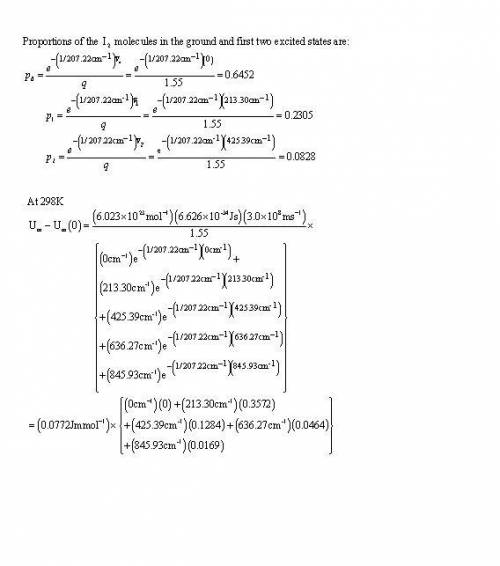
Calculate, by explicit summation, the vibrational partition functionand the vibrational contribution to the molar internal energy of I2 Molecule At (a) 100 K, (b) 298 K given that its vibrational energy levels lie at thefollowing wavenumbers above the zero-point energy level: 0, 213.30, 425.39,636.27, 845.93 cm−1. What proportion of I2molecules are in the ground andfirst two excited levels at the two temperatures

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

You know the right answer?
Calculate, by explicit summation, the vibrational partition functionand the vibrational contribution...
Questions

Mathematics, 12.05.2021 04:00


Mathematics, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00




Mathematics, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00




Biology, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00


Mathematics, 12.05.2021 04:00


Mathematics, 12.05.2021 04:00

English, 12.05.2021 04:00