ASAP WILL GIVE BRAINLIEST
Write a balanced chemical equation for the following
chemical...

Chemistry, 22.04.2020 03:54 59bobolucci
ASAP WILL GIVE BRAINLIEST
Write a balanced chemical equation for the following
chemical reaction.
Solid magnesium reacts with aqueous copper (II) nitrate to form aqueous
magnesium nitrate and solid copper

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Questions



English, 20.04.2021 19:20

Mathematics, 20.04.2021 19:20





Mathematics, 20.04.2021 19:20

Social Studies, 20.04.2021 19:20

History, 20.04.2021 19:20

Social Studies, 20.04.2021 19:20



Health, 20.04.2021 19:20

Mathematics, 20.04.2021 19:20

Biology, 20.04.2021 19:20



Computers and Technology, 20.04.2021 19:20

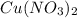 (aq) →
(aq) → 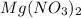 (aq) + Cu(s)
(aq) + Cu(s)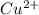 ) and nitrate (
) and nitrate (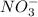 ). It will create:
). It will create: 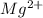 ) and nitrate (
) and nitrate (

