

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

You know the right answer?
A sample of air contains 78.08% nitrogen, 20.94% oxygen, 0.0500% carbon dioxide, and 0.930% argon by...
Questions


Mathematics, 17.09.2019 03:00





Biology, 17.09.2019 03:00



Mathematics, 17.09.2019 03:00


Mathematics, 17.09.2019 03:00

Biology, 17.09.2019 03:00

History, 17.09.2019 03:00

English, 17.09.2019 03:00


Mathematics, 17.09.2019 03:00



Mathematics, 17.09.2019 03:00

 ,
, 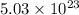 ,
,  and
and 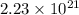 respectively.
respectively.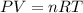
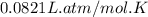
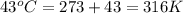
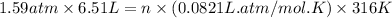
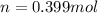
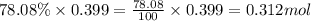
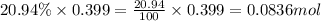
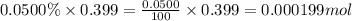
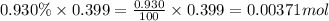
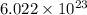 number of molecules of nitrogen.
number of molecules of nitrogen.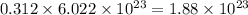 number of molecules of nitrogen.
number of molecules of nitrogen.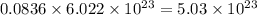 number of molecules of oxygen.
number of molecules of oxygen.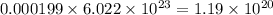 number of molecules of carbon dioxide.
number of molecules of carbon dioxide.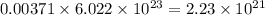 number of molecules of argon.
number of molecules of argon.

