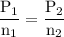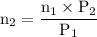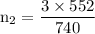Chemistry, 22.04.2020 03:59 davelopez979
A catalytic converter in an automobile uses a palladium or platinum catalyst to convert carbon monoxide gas to carbon dioxide according to the following reaction: 2CO(g) + O2 (g) --> 2CO2(g) A chemist researching the effectiveness of a new catalyst combines a 2.0 : 1.0 mole ratio mixture of carbon monoxide and oxygen gas (respectively) over the catalyst in a 2.45L flask at a total pressure of 740 torr and a temperature of 552 C . When the reaction is complete, the pressure in the flask has dropped to 552 torr. What percentage of the carbon monoxide was converted to carbon dioxide?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

You know the right answer?
A catalytic converter in an automobile uses a palladium or platinum catalyst to convert carbon monox...
Questions

Mathematics, 17.11.2020 08:50

Health, 17.11.2020 08:50

Mathematics, 17.11.2020 08:50



Mathematics, 17.11.2020 08:50

Physics, 17.11.2020 08:50



Mathematics, 17.11.2020 08:50


English, 17.11.2020 08:50

Mathematics, 17.11.2020 08:50

Mathematics, 17.11.2020 08:50



Mathematics, 17.11.2020 08:50


Mathematics, 17.11.2020 08:50

 2CO₂
2CO₂