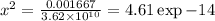Chemistry, 22.04.2020 04:27 laylay7383
G Using the complex based titration system: 50.00 mL 0.00250 M Ca2 titrated with 0.0050 M EDTA, buffered at pH 11.0 determine (i) first pCa first before initiating the titration process and then (ii) at equivalence when all the Ca2 is titrated to CaY2-. Please, use your text books and/or lecture notes to find potentially missing information about constants needed to solve the problem.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
G Using the complex based titration system: 50.00 mL 0.00250 M Ca2 titrated with 0.0050 M EDTA, buff...
Questions


Mathematics, 14.07.2019 02:00



Mathematics, 14.07.2019 02:00





Mathematics, 14.07.2019 02:00



Mathematics, 14.07.2019 02:00




Mathematics, 14.07.2019 02:00


English, 14.07.2019 02:00

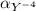 =0.81
=0.81 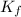 =0.81x 4.47 x10¹⁰
=0.81x 4.47 x10¹⁰![[CaY^{2-}] = \frac{Initial,moles,of, Ca^{2+}}{Total,Volume} = \frac{0.125mol}{(50.00+25.00)mL} = 0.001667M](/tpl/images/0617/6168/49a70.png)
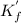
![{K^'}_f = \frac{[CaY^{2-}]}{[Ca^{2+}][Y^4]}=\frac{0.001667-x}{x.x} =\frac{0.001667-x}{x^2}\\\\x^2 = \frac{0.001667-x}{{K^'}_f}\\ \\](/tpl/images/0617/6168/d054e.png)