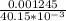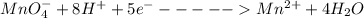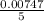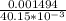Chemistry, 22.04.2020 04:39 aroland1990x
A Cu2+ solution is prepared by dissolving a 0.4749 g piece of copper wire in acid. The solution is then passed through a Walden reductor, reducing Cu2+ to Cu+ . The resulting Cu+ solution required 40.15 mL of each of the titrants to reach the endpoint. Calculate the concentration of each titrant.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
A Cu2+ solution is prepared by dissolving a 0.4749 g piece of copper wire in acid. The solution is t...
Questions


Mathematics, 17.07.2019 11:00

History, 17.07.2019 11:00

English, 17.07.2019 11:00

Mathematics, 17.07.2019 11:00



Mathematics, 17.07.2019 11:00

Biology, 17.07.2019 11:00


History, 17.07.2019 11:00


Health, 17.07.2019 11:00


Mathematics, 17.07.2019 11:00


Mathematics, 17.07.2019 11:00

Biology, 17.07.2019 11:00

Biology, 17.07.2019 11:00

Mathematics, 17.07.2019 11:00

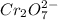 = 0.03101 M
= 0.03101 M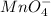 = 0.03721 M
= 0.03721 M
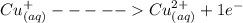
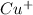 = 1 mole of
= 1 mole of 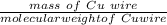
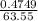
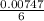
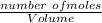
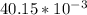 ; we have:
; we have: