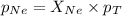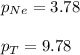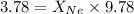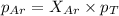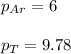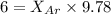Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

You know the right answer?
A vessel containing Ne(g) and Ar(g) has a total pressure of 9.78. If the partial pressure of the Neo...
Questions

Biology, 29.06.2019 06:00

Biology, 29.06.2019 06:00

Health, 29.06.2019 06:00



History, 29.06.2019 06:00

Mathematics, 29.06.2019 06:00

English, 29.06.2019 06:00

History, 29.06.2019 06:00

Mathematics, 29.06.2019 06:00

History, 29.06.2019 06:00


Biology, 29.06.2019 06:00

Chemistry, 29.06.2019 06:00


Arts, 29.06.2019 06:00

Social Studies, 29.06.2019 06:00



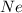 and
and 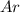 is, 0.387 and 0.613 respectively.
is, 0.387 and 0.613 respectively.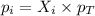
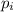 = partial pressure of gas
= partial pressure of gas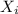 = mole fraction of gas
= mole fraction of gas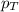 = total pressure of gas
= total pressure of gas