
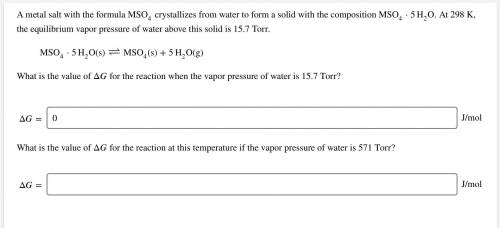
A metal salt with the formula MSO4 crystallizes from water to form a solid with the composition MSO4⋅5H2O. At 298 K, the equilibrium vapor pressure of water above this solid is 15.7 Torr.
MSO4⋅5H2O(s)↽−−⇀MSO4(s)+5H2O(g)
What is the value of ΔG for the reaction when the vapor pressure of water is 15.7 Torr?
Δ= ? J/mol
What is the value of Δ for the reaction when the vapor pressure of water is 571 Torr?
ΔG= ? J/mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2


Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3
You know the right answer?
A metal salt with the formula MSO4 crystallizes from water to form a solid with the composition MSO4...
Questions









Mathematics, 17.10.2019 02:10



Mathematics, 17.10.2019 02:10


Mathematics, 17.10.2019 02:10

History, 17.10.2019 02:10







