
Chemistry, 22.04.2020 16:34 isaiahmichel93081
A solution is prepared by dissolving 15.0 g of NH3 in 250 g of water. The density of the resulting solution is 0.974 g/mL. The mole fraction of NH3 in the solution is .A) 16.8B) 0.940C) 0.0597D) 0.922E) 0.0640

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
A solution is prepared by dissolving 15.0 g of NH3 in 250 g of water. The density of the resulting s...
Questions

Physics, 05.05.2020 15:40


Mathematics, 05.05.2020 15:40


Mathematics, 05.05.2020 15:40


Mathematics, 05.05.2020 15:40

Mathematics, 05.05.2020 15:40

Mathematics, 05.05.2020 15:40




Mathematics, 05.05.2020 15:40



Mathematics, 05.05.2020 15:40



Chemistry, 05.05.2020 15:40

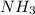 in solution is 0.0597.
in solution is 0.0597.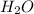 = 18.015 g/mol
= 18.015 g/mol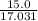 moles of
moles of 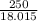 moles of
moles of 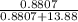 = 0.0597
= 0.0597

