
Chemistry, 22.04.2020 19:00 arieannaensley0616
Given the balanced equation 2C4H10 + 13O2 → 8CO2 + 10H2O, how many moles of CO2 are produced when 14.9g of O2 are used?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2


Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Given the balanced equation 2C4H10 + 13O2 → 8CO2 + 10H2O, how many moles of CO2 are produced when 14...
Questions

Mathematics, 08.08.2021 17:00




English, 08.08.2021 17:00

Mathematics, 08.08.2021 17:00

Mathematics, 08.08.2021 17:00

Mathematics, 08.08.2021 17:00




Business, 08.08.2021 17:10

Engineering, 08.08.2021 17:10

Mathematics, 08.08.2021 17:10


Biology, 08.08.2021 17:10



Advanced Placement (AP), 08.08.2021 17:10

Mathematics, 08.08.2021 17:10

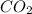 produced are, 0.287 moles.
produced are, 0.287 moles.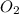 = 14.9 g
= 14.9 g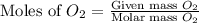
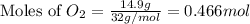
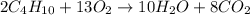
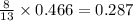 mole of
mole of 

