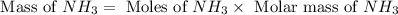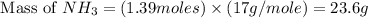Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1
You know the right answer?
When 100.g Mg3N2 reacts with 75.0 g H2O, what is the maximum theoretical yield of NH3?
M...
M...
Questions

Mathematics, 09.11.2020 17:00





Advanced Placement (AP), 09.11.2020 17:00














Mathematics, 09.11.2020 17:00

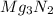 = 100.0 g
= 100.0 g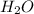 = 75.0 g
= 75.0 g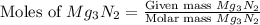
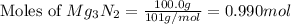
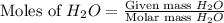
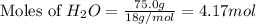
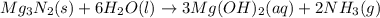
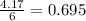 moles of
moles of 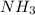
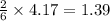 mole of
mole of