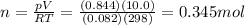How many moles of oxygen must be in a 10.0 L container to exert a pressure of
0.844 atm at a t...


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2


Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

You know the right answer?
Questions



Spanish, 19.10.2019 22:30



History, 19.10.2019 22:30

Mathematics, 19.10.2019 22:30

Mathematics, 19.10.2019 22:30

Arts, 19.10.2019 22:30











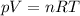
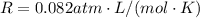 is the gas constant
is the gas constant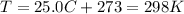 is the absolute temperature of the gas
is the absolute temperature of the gas