
Chemistry, 22.04.2020 22:08 tdahna0403
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product, phosgene (COCl2 ) CO(g) Cl2 (g) COCl2 (g) If the equilibrium constant for this reaction is Kc = 248, predict, if possible, what will happen when the reactants and product are combined with the concentrations shown. [CO] = [Cl2] = 0.010 M; [COCl2] = 0.070 M

Answers: 3
Another question on Chemistry




Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product,...
Questions



English, 31.03.2020 03:23


History, 31.03.2020 03:23


Chemistry, 31.03.2020 03:23





Mathematics, 31.03.2020 03:23




Mathematics, 31.03.2020 03:23




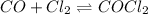
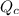 ) for this reaction is represented as:
) for this reaction is represented as:![Q_{c}=\frac{[COCl_{2}]}{[CO][Cl_{2}]}](/tpl/images/0619/3924/9faba.png)
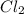 ] = 0.010 M and [
] = 0.010 M and [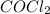 ] = 0.070 M
] = 0.070 M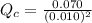 = 700
= 700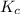 therefore reaction will proceed towards forward direction i.e. more
therefore reaction will proceed towards forward direction i.e. more

