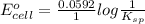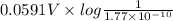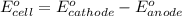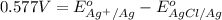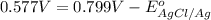Chemistry, 22.04.2020 22:03 Buttercream16
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the solubility product constant for AgCl at 298 K is 1.77 × 10 − 10 and the standard reduction potential of the half‑reaction Ag + ( aq ) + e − − ⇀ ↽ − Ag ( s ) is + 0.799 V .

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the...
Questions

Chemistry, 24.10.2020 07:50


Mathematics, 24.10.2020 07:50

Social Studies, 24.10.2020 07:50

History, 24.10.2020 07:50

Mathematics, 24.10.2020 07:50

Social Studies, 24.10.2020 07:50


Physics, 24.10.2020 07:50

Biology, 24.10.2020 07:50

Advanced Placement (AP), 24.10.2020 07:50

Mathematics, 24.10.2020 07:50

Physics, 24.10.2020 07:50

English, 24.10.2020 07:50

Business, 24.10.2020 07:50

Mathematics, 24.10.2020 07:50

Mathematics, 24.10.2020 07:50


Mathematics, 24.10.2020 07:50

Mathematics, 24.10.2020 07:50

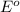 for the half-cell reaction is 0.222 V.
for the half-cell reaction is 0.222 V.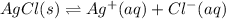
![K_{sp} = [Ag^{+}][Cl^{-}]](/tpl/images/0619/3632/031c0.png)
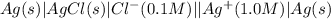
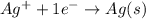 ,
, 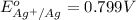
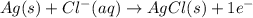 ,
, 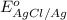 = ?
= ?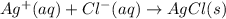
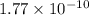
![E_{cell} = E^{o}_{cell} - \frac{0.0592 V}{n} log \frac{[AgCl]}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/d09c5.png)
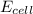 = 0.00 V
= 0.00 V![0.00 = E^{o}_{cell} - \frac{0.0592 V}{1} log \frac{1}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/1250d.png)