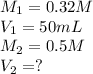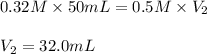The reaction of hydrochloric acid (HCl) with ammonia (NH3) is described by the equation:
...

The reaction of hydrochloric acid (HCl) with ammonia (NH3) is described by the equation:
HCl + NH3 → NH4Cl
A student is titrating 50 mL of 0.32 M NH3 with 0.5 M HCl. How much hydrochloric acid must be added to react completely with the ammonia?
A. 6.4 mL
B. 16.0 mL
C. 32.0 mL
D. 50.0 mL

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Questions

Biology, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00



Chemistry, 24.02.2021 02:00

Chemistry, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00


Social Studies, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00


Mathematics, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00


Mathematics, 24.02.2021 02:00



Mathematics, 24.02.2021 02:00

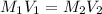
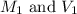 are the initial molarity and volume of NH₃.
are the initial molarity and volume of NH₃.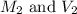 are the final molarity and volume of HCl.
are the final molarity and volume of HCl.