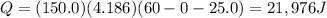CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(...

Chemistry, 22.04.2020 23:16 ethangorrell67
CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(s), has been proposed as a substance that can be used to heat water quickly for portable heating packs or for cooking. When placed in water, Cao(s) reacts as shown by the equation above.
A student wants to design a heating pad that could heat a 150.0 g sample of water from 25.0°C to 60.0°C.
Calculate the amount of heat, in joules, that the water must absorb for its
temperature to change by this amount. (Assume that the specific heat capacity
of the water is 4.18 J/gK).

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 09:00
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions represents an exothermic reaction? nh3(g) + 12.0 kcal ½n2(g) + 3/2 h2(g) ch4 + 2o2 co2 + 2h2o + 212,800 cal c + 2s cs2, h = 27,550 cal c(graphite) c(diamond), h = 0.45 kcal 2h2o 2h2 + o2, h = +58 kcal
Answers: 1

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1
You know the right answer?
Questions







History, 10.02.2020 21:26


History, 10.02.2020 21:26


Computers and Technology, 10.02.2020 21:26

Biology, 10.02.2020 21:26

History, 10.02.2020 21:26

Mathematics, 10.02.2020 21:26

Geography, 10.02.2020 21:26



English, 10.02.2020 21:26


Geography, 10.02.2020 21:26

 , the amount of heat that must be supplied to the substance must be:
, the amount of heat that must be supplied to the substance must be: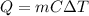
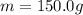 is the mass
is the mass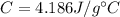 is the specific heat capacity of water
is the specific heat capacity of water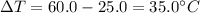 is the increase in temperature
is the increase in temperature