Chemistry, 22.04.2020 23:46 micahatwood03
After the completion of a gas forming reaction, the column of water remaining in the collection flask was measured to me 55 mm high, the vapor pressure of water is 0.0313 atm at 25oC, and the atmospheric pressure that day was measured as 0.950 atm, what is the partial pressure of the gas produced by the reaction

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
After the completion of a gas forming reaction, the column of water remaining in the collection flas...
Questions

Chemistry, 31.03.2021 01:00





History, 31.03.2021 01:00



Geography, 31.03.2021 01:00





Mathematics, 31.03.2021 01:00

Mathematics, 31.03.2021 01:00

Mathematics, 31.03.2021 01:00


Mathematics, 31.03.2021 01:00


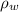 = 0.0313 atm
= 0.0313 atm 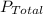 = 0.950 atm
= 0.950 atm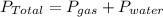
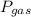 =
=