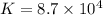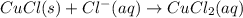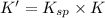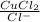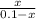The copper(I) ion forms a chloride salt (CuCl) that has Ksp = 1.2 x 10-6. Copper(I) also forms a complex ion with Cl-:Cu+ (aq) + 2Cl- (aq) ⇄ CuCl2- (aq) K = 8.7 x 104(a) Calculate the solubility of CuCl in pure water. (Ignore CuCl2- formation for part a).(b) Calculate the solubility of CuCl in 0.100 M NaCl solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3

Chemistry, 23.06.2019 19:30
Why does 4.03/0.0000035 = 1.2 x 106, instead of a different number of significant figures?
Answers: 1
You know the right answer?
The copper(I) ion forms a chloride salt (CuCl) that has Ksp = 1.2 x 10-6. Copper(I) also forms a com...
Questions

Biology, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30



Arts, 28.05.2021 19:30




Mathematics, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30




Mathematics, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30

History, 28.05.2021 19:30


Mathematics, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30

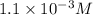 .
.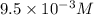 .
.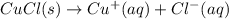
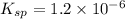
![K_{sp} = [Cu^{+}][Cl^{-}]](/tpl/images/0619/9671/6e8cf.png)
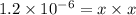
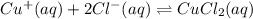 ,
,