

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
The Ksp of Al(OH)3 at 25 oC is 1 x 10-33. Consider a solution that is 1.0 x 10-10 M Al(NO3)3 and 1.0...
Questions


Mathematics, 18.09.2021 04:30


Mathematics, 18.09.2021 04:30


Mathematics, 18.09.2021 04:30



English, 18.09.2021 04:30




Mathematics, 18.09.2021 04:30

Mathematics, 18.09.2021 04:30


Mathematics, 18.09.2021 04:30



Mathematics, 18.09.2021 04:40

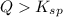 and a precipitate will form.
and a precipitate will form.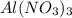 =
= 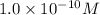
 =
= 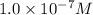
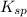 of
of 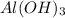 =
= 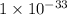
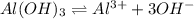
![Q=[Al^{3+}][OH^-]^3](/tpl/images/0619/9788/355c8.png)
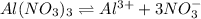
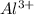 =
= 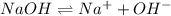
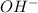 =
= 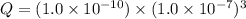
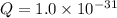
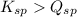 ; the reaction is product favored. (No precipitation)
; the reaction is product favored. (No precipitation)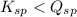 ; the reaction is reactant favored. (Precipitation)
; the reaction is reactant favored. (Precipitation)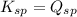 ; the reaction is in equilibrium. (Sparingly soluble)
; the reaction is in equilibrium. (Sparingly soluble)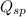 is more than
is more than 

