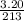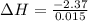Chemistry, 23.04.2020 02:56 LindaCat78
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate placed into 103.2 mL of water. The temperature of the solution is initially at 23.2 oC. After the reaction takes place, the temperature of the solution is 17.7 oC. How much heat was absorbed or lost by the surroundings? Use 4.184 J/goC for the specific heat of the solution. Put your answer in units of kJ and make sure the sign is correct. What would be the enthalpy for the dissolution reaction of one mole of aluminum nitrate? Put your answer in kJ/mol and watch the sign for the enthalpy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate place...
Questions







Mathematics, 20.04.2020 20:25

History, 20.04.2020 20:25

Mathematics, 20.04.2020 20:25

Mathematics, 20.04.2020 20:25



Mathematics, 20.04.2020 20:25




Mathematics, 20.04.2020 20:26


Mathematics, 20.04.2020 20:26

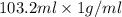
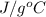 .
.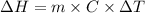
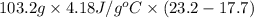
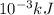 . So, 2372.5 J will be converted into kJ as follows.
. So, 2372.5 J will be converted into kJ as follows.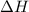 = 2.37 kJ
= 2.37 kJ 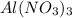 = 213 g/mol
= 213 g/mol