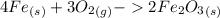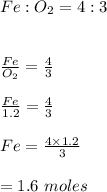Chemistry, 23.04.2020 05:20 tiffanydowell13
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 mol of oxygen to produce iron(III) oxide. His calculation is shown below: 1.20

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 m...
Questions

English, 07.12.2021 05:10



Mathematics, 07.12.2021 05:10

Mathematics, 07.12.2021 05:10

Mathematics, 07.12.2021 05:10



English, 07.12.2021 05:10


Mathematics, 07.12.2021 05:10


Mathematics, 07.12.2021 05:10

Physics, 07.12.2021 05:10

Mathematics, 07.12.2021 05:10




World Languages, 07.12.2021 05:10

History, 07.12.2021 05:10