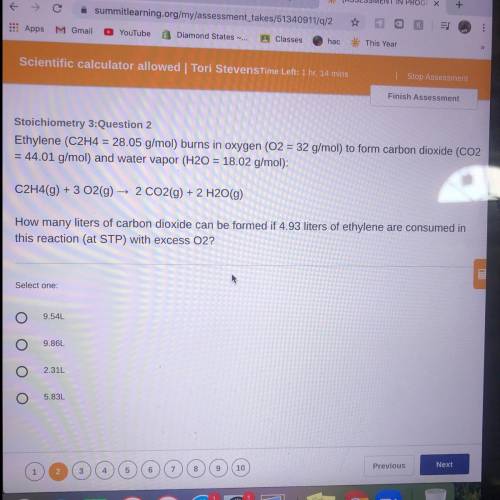
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44...

Chemistry, 23.04.2020 20:23 tchase0616
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44.01 g/mol) and water vapor (H20 = 18.02 g/mol):
C2H4(9) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
How many liters of carbon dioxide can be formed if 4.93 liters of ethylene are consumed in
this reaction (at STP) with excess O2?
Select one:
9.54L
9.86L
2.31L
5.83L

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1
You know the right answer?
Questions


Social Studies, 31.12.2020 14:00

Biology, 31.12.2020 14:00

History, 31.12.2020 14:00

Mathematics, 31.12.2020 14:00

Social Studies, 31.12.2020 14:00


Social Studies, 31.12.2020 14:00

Mathematics, 31.12.2020 14:00


Mathematics, 31.12.2020 14:00



Health, 31.12.2020 14:00

Mathematics, 31.12.2020 14:00


Mathematics, 31.12.2020 14:00

English, 31.12.2020 14:00


Health, 31.12.2020 14:00



