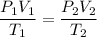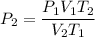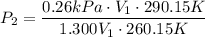Chemistry, 23.04.2020 22:23 christiantorres57
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of -161. °C. Suppose the temperature of a sample of methane gas is raised from -13.0 °C to 17.0°C, and at the same time the pressure is changed. If the initial pressure was 0.26 kPa and the volume increased by 30.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions

Mathematics, 16.10.2020 17:01


English, 16.10.2020 17:01


History, 16.10.2020 17:01

English, 16.10.2020 17:01

Physics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

English, 16.10.2020 17:01



Biology, 16.10.2020 17:01


Geography, 16.10.2020 17:01



Mathematics, 16.10.2020 17:01

Chemistry, 16.10.2020 17:01

Social Studies, 16.10.2020 17:01

History, 16.10.2020 17:01