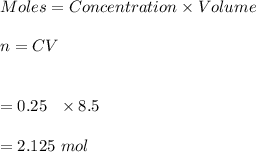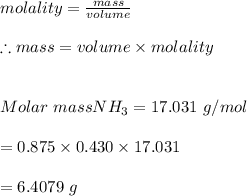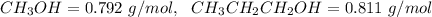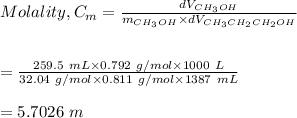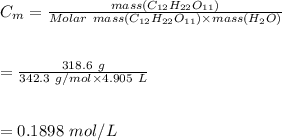Chemistry, 24.04.2020 01:00 robert7248
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric acid solution
2. 7.300 moles of sodium nitrite are needed for a reaction. The solution is 5.450 M. How many mL are needed?
3. What mass (in g) of NH3 must be dissolved in 875 g of methanol to make a 0.430 molal solution?
4. Calculate the molality of a solution that is prepared by mixing 259.5 mL of CH3OH
(d = 0.792 g/mL) and 1387 mL of CH3CH2CH2OH (d = 0.811 g/mL)
5. A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the molarity of the solution

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric ac...
Questions

Mathematics, 12.12.2019 12:31

Mathematics, 12.12.2019 12:31


Chemistry, 12.12.2019 12:31


Mathematics, 12.12.2019 12:31

Spanish, 12.12.2019 12:31


History, 12.12.2019 12:31






Mathematics, 12.12.2019 12:31


English, 12.12.2019 12:31

Health, 12.12.2019 12:31


History, 12.12.2019 12:31