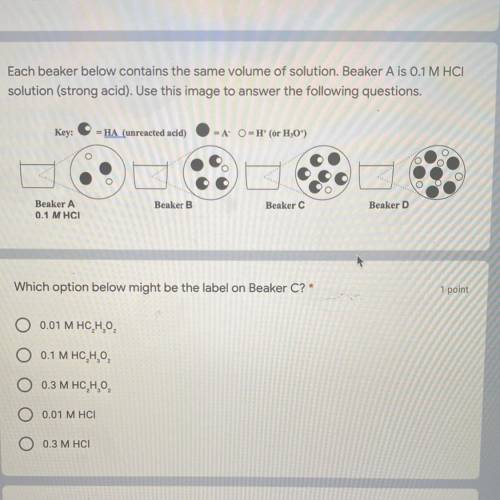
Each beaker below contains the same volume of solution. Beaker A is 0.1 M HCI
solution (strong...

Chemistry, 24.04.2020 03:43 redhot12352
Each beaker below contains the same volume of solution. Beaker A is 0.1 M HCI
solution (strong acid). Use this image to answer the following questions.
Key:
-HA (unreacted acid)
-A
-H(or H,0")
Beaker.
Beaker B
Beaker C
Beaker A
0.1 MHCI
Beaker D
Which option below might be the label on Beaker C?'
1 point
O 0.01 M HC, H,O,
O 0.1 M HC, H,O,
O 0.3 M HC, H,O,
O 0.01 M HCI
O 0.3 M HCI

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
Questions

Mathematics, 05.08.2019 02:00

Chemistry, 05.08.2019 02:00

History, 05.08.2019 02:00

History, 05.08.2019 02:00









Health, 05.08.2019 02:00


Biology, 05.08.2019 02:00

English, 05.08.2019 02:00


Mathematics, 05.08.2019 02:00

History, 05.08.2019 02:00



