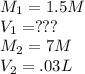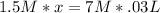Chemistry, 24.04.2020 06:39 brownvester44
PLS ANSWER ASAP, WILL GIVE BRAINLIEST ANSWER
Calculate the volume of 1.5 M nitric acid required to neutralize 30.0 mL of 7.0 M sodium hydroxide.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
PLS ANSWER ASAP, WILL GIVE BRAINLIEST ANSWER
Calculate the volume of 1.5 M nitric acid require...
Calculate the volume of 1.5 M nitric acid require...
Questions

Arts, 01.04.2021 18:10


Mathematics, 01.04.2021 18:10



Mathematics, 01.04.2021 18:10

Physics, 01.04.2021 18:10

Mathematics, 01.04.2021 18:10

Mathematics, 01.04.2021 18:10


English, 01.04.2021 18:10

Social Studies, 01.04.2021 18:10



History, 01.04.2021 18:10


Law, 01.04.2021 18:10

Geography, 01.04.2021 18:10

Mathematics, 01.04.2021 18:10

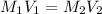 . M stands for molarity of the given substance, and V stands for the volume that the substance occupies.
. M stands for molarity of the given substance, and V stands for the volume that the substance occupies.