Chemistry, 24.04.2020 15:18 spoo262005
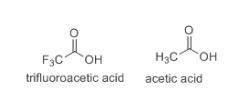
Which of the following item(s) explain the differences between the Ka values. Choose one or more: A. The negative charge is on the more electronegative fluorine atom in trifluoroacetate. B. The oxidation state for oxygen in trifluoroacetate is more negative than the oxidation state for oxygen in acetate. C. The trifluoroacetate molecule has more resonance structures than the acetate molecule. D. The electron-withdrawing fluorine atoms pull electron density from the oxygen in trifluoroacetate. The negative charge is more stabilized in trifluoroacetate by this effect.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Which of the following item(s) explain the differences between the Ka values. Choose one or more: A....
Questions