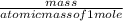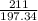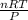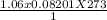1. Zinc metal reacts with 100 mL of 6.00 M H2SO4. How many grams of Zinc Sulfate are produced and how many liters of Hydrogen gas would be released?
2.A 211 g sample of BaCO3 is reacted with HNO3. Assuming the acid is present is excess, what mass and volume of CO2 gas will be produced?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
1. Zinc metal reacts with 100 mL of 6.00 M H2SO4. How many grams of Zinc Sulfate are produced and ho...
Questions

Mathematics, 04.08.2020 14:01




Mathematics, 04.08.2020 14:01

Social Studies, 04.08.2020 14:01



Chemistry, 04.08.2020 14:01

Computers and Technology, 04.08.2020 14:01





History, 04.08.2020 14:01


Computers and Technology, 04.08.2020 14:01

Mathematics, 04.08.2020 14:01

Mathematics, 04.08.2020 14:01

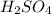 ⇒ ZnS
⇒ ZnS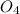 +
+ 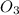 + 2HN
+ 2HN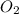 +
+ 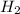 O
O