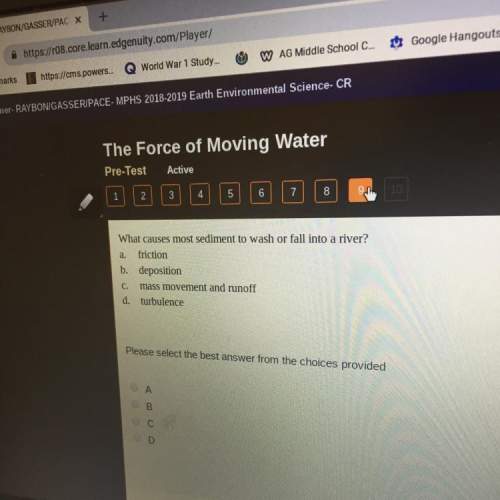
Chemistry, 25.04.2020 00:39 almendares55
Potassium hydroxide is very soluble in water, resulting in extremely basic solutions. A 121g sample KOH is dissolved in water at 25∘C to make up 100.0mL of solution. The molar mass of KOH is 56.11gmol. What is the pH of the solution at 25.0∘C? Round the answer to two decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Potassium hydroxide is very soluble in water, resulting in extremely basic solutions. A 121g sample...
Questions



History, 25.06.2021 14:30

Social Studies, 25.06.2021 14:30

Mathematics, 25.06.2021 14:30

Geography, 25.06.2021 14:30


Law, 25.06.2021 14:30

Advanced Placement (AP), 25.06.2021 14:30

Mathematics, 25.06.2021 14:30



Mathematics, 25.06.2021 14:30

History, 25.06.2021 14:30


Biology, 25.06.2021 14:50

Chemistry, 25.06.2021 14:50

Mathematics, 25.06.2021 14:50


Social Studies, 25.06.2021 14:50