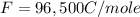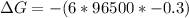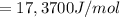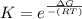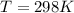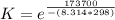Chemistry, 25.04.2020 03:33 madelynlittle5399
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and Cr3 (aq) to give Cr(s) and Fe2 (aq). Give your answer using E-notation with NO decimal places (e. g., 2 x 10-2 would be 2E-2; and 2.12 x 10-2 would also be 2E-2.). Do NOT include spaces, units, punctuation or anything else silly! Use the reduction potentials for Cr3 (aq) is -0.74 V and for Fe2 (aq) is -0.440 V. [a]

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and...
Questions





Mathematics, 08.09.2021 22:30


Mathematics, 08.09.2021 22:30


Physics, 08.09.2021 22:30

Chemistry, 08.09.2021 22:30


Chemistry, 08.09.2021 22:30

Chemistry, 08.09.2021 22:30



Mathematics, 08.09.2021 22:30

Mathematics, 08.09.2021 22:30


Mathematics, 08.09.2021 22:30

English, 08.09.2021 22:30

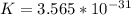
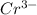 =
= 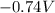
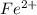 =
= 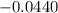

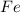 is oxidized to
is oxidized to 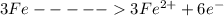
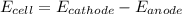
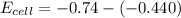
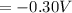
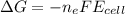
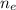 is the number of moles of electron which is 6
is the number of moles of electron which is 6