
Chemistry, 25.04.2020 03:00 destinywashere101
A chemist titrates 216.8 mL of a 0.3200 M butanoic acid (HC3H7CO2) solution with a 0.3200 M solution of NaOH. The titration setup shows that the butanoic acid solution is in the (Erlenmeyer) flask, and the NaOH solution is in the burette. The pKa of butanoic acid is 4.82. Calculate the pH of the solution in the flask after the chemist has added 231.4 mL of NaOH solution to it.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
A chemist titrates 216.8 mL of a 0.3200 M butanoic acid (HC3H7CO2) solution with a 0.3200 M solution...
Questions




Spanish, 26.03.2020 02:01

History, 26.03.2020 02:01

Mathematics, 26.03.2020 02:01


Mathematics, 26.03.2020 02:01







Social Studies, 26.03.2020 02:01

Mathematics, 26.03.2020 02:01


Mathematics, 26.03.2020 02:01

Computers and Technology, 26.03.2020 02:01

Biology, 26.03.2020 02:01

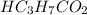 = 0.32 M,
= 0.32 M,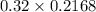
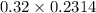
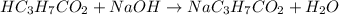
![[OH^{-}] = \frac{n}{V}](/tpl/images/0626/3976/691f7.png)
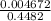
![-log [OH^{-}]](/tpl/images/0626/3976/337a4.png)


