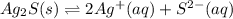Chemistry, 25.04.2020 03:24 zanestone12
For the reaction Ag2S(s) ⇌ 2Ag+(aq) + S2−(aq), what happens to the equilibrium position if the amount of silver ion is halved?For the reaction S(s) ⇌ 2(aq) + (aq), what happens to the equilibrium position if the amount of silver ion is halved?

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
For the reaction Ag2S(s) ⇌ 2Ag+(aq) + S2−(aq), what happens to the equilibrium position if the amoun...
Questions

Mathematics, 11.11.2020 03:20

History, 11.11.2020 03:20

World Languages, 11.11.2020 03:20

Mathematics, 11.11.2020 03:20


Mathematics, 11.11.2020 03:20






Mathematics, 11.11.2020 03:20


English, 11.11.2020 03:20

Chemistry, 11.11.2020 03:20


History, 11.11.2020 03:20



History, 11.11.2020 03:20