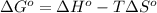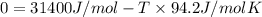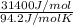Chemistry, 25.04.2020 03:48 oliviahopebigel
Chloroform, formerly used as an anaesthetic and now believed to be a carcinogen, has a heat of vaporization ΔHvaporization = 31.4 kJ mol-1. The change, CHCl3(l) CHCl3(g) has ΔSº = 94.2 J mol-1 K-1. At what temperature do we expect CHCl3 to boil (i. e., at what temperature will liquid and vapor be in equilibrium at 1 atm pressure)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Chloroform, formerly used as an anaesthetic and now believed to be a carcinogen, has a heat of vapor...
Questions




Mathematics, 01.04.2021 17:50


English, 01.04.2021 17:50

English, 01.04.2021 17:50

Mathematics, 01.04.2021 17:50


Mathematics, 01.04.2021 17:50

Mathematics, 01.04.2021 17:50



History, 01.04.2021 17:50






History, 01.04.2021 17:50

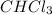 to boil.
to boil.