Chemistry, 25.04.2020 04:09 hellodarkness14
A piece of copper (12.0 g) is heated to 100.0 °C. A piece of chromium (also 12.0 g) is chilled in an ice bath to 0 °C. The specific heat capacity of water is 4.184 J/g ⋅°C.
Both pieces of metal are placed in a beaker containing 200.0 g at 20.0 °C.
(a) Will the temperature of the water be greater than or less than 20.0 °C when thermal equilibrium is reached?
(b) Both pieces of metal are placed in a beaker containing 200.0 g at 20.0 °C. Calculate the final temperature.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
A piece of copper (12.0 g) is heated to 100.0 °C. A piece of chromium (also 12.0 g) is chilled in an...
Questions



Mathematics, 13.10.2020 01:01


Social Studies, 13.10.2020 01:01


Health, 13.10.2020 01:01


English, 13.10.2020 01:01



History, 13.10.2020 01:01



Social Studies, 13.10.2020 01:01

English, 13.10.2020 01:01

History, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01

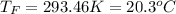
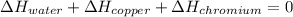
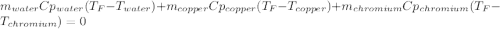 In such a way, by knowing that the heat capacities of copper and chromium are 0.386 and 0.45 J/(g°C) respectively, by solving for the equilibrium temperature one has:
In such a way, by knowing that the heat capacities of copper and chromium are 0.386 and 0.45 J/(g°C) respectively, by solving for the equilibrium temperature one has: