Chemistry, 25.04.2020 04:49 jessieeverett432
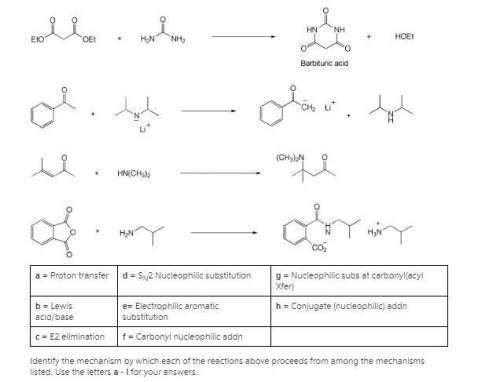
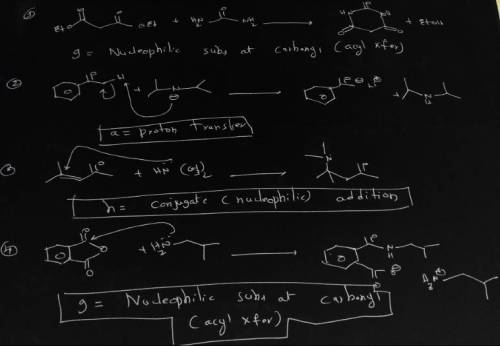
A = Proton transfer d = SN2 Nucleophilic substitution g = Nucleophilic subs at carbonyl(acyl Xfer) b = Lewis acid/base e= Electrophilic aromatic substitution h = Conjugate (nucleophilic) addn c = E2 elimination f = Carbonyl nucleophilic addn Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. n(nh3)3

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
A = Proton transfer d = SN2 Nucleophilic substitution g = Nucleophilic subs at carbonyl(acyl Xfer) b...
Questions


Social Studies, 30.01.2021 08:30

Mathematics, 30.01.2021 08:30

Mathematics, 30.01.2021 08:30

History, 30.01.2021 08:30

Mathematics, 30.01.2021 08:30

Mathematics, 30.01.2021 08:30


Health, 30.01.2021 08:30


Mathematics, 30.01.2021 08:30

Social Studies, 30.01.2021 08:30

Spanish, 30.01.2021 08:40


English, 30.01.2021 08:40



Biology, 30.01.2021 08:40

English, 30.01.2021 08:40

Mathematics, 30.01.2021 08:40