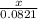Ca(ClO3)2→ CaCl2 + O2
What mass of O2 results from the decomposition of 17.00 g of calcium ch...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Questions



Arts, 18.01.2021 21:30

Mathematics, 18.01.2021 21:30


English, 18.01.2021 21:30




Social Studies, 18.01.2021 21:30





English, 18.01.2021 21:30

Physics, 18.01.2021 21:30


Mathematics, 18.01.2021 21:30


 )
) ⇒
⇒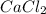 + 3
+ 3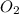
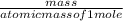
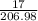
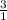 =
=