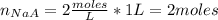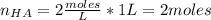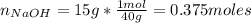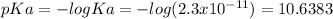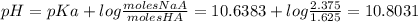Chemistry, 25.04.2020 06:49 philkas414
One liter of a buffer contains 2.00 Molar NaA and 2.00 Molar HA. (A- is the anion of an acid) 15.00 g NaOH is added. (Assume no volume change) What is the new pH?
HA (aq) + H2O (l) A - (aq) + H3O+ (aq) Ka = 2.3 x 10-11

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
One liter of a buffer contains 2.00 Molar NaA and 2.00 Molar HA. (A- is the anion of an acid) 15.00...
Questions

Mathematics, 11.11.2019 21:31


Physics, 11.11.2019 21:31



Mathematics, 11.11.2019 21:31

Physics, 11.11.2019 21:31








History, 11.11.2019 21:31



English, 11.11.2019 21:31