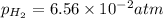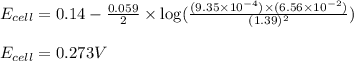Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
What is the calculated value of the cell potential at 298K for an
electrochemical cell with th...
electrochemical cell with th...
Questions

Mathematics, 26.06.2021 01:00

History, 26.06.2021 01:00

Mathematics, 26.06.2021 01:00


Mathematics, 26.06.2021 01:00


Computers and Technology, 26.06.2021 01:00

Mathematics, 26.06.2021 01:00


Mathematics, 26.06.2021 01:00

English, 26.06.2021 01:00

Computers and Technology, 26.06.2021 01:00



English, 26.06.2021 01:00



Mathematics, 26.06.2021 01:00

Mathematics, 26.06.2021 01:00

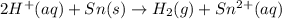
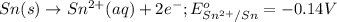
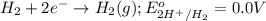
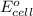 of the reaction, we use the equation:
of the reaction, we use the equation: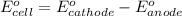
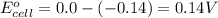
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Sn^{2+}]\times p_{H_2}}{[H^+]^2}](/tpl/images/0627/3491/76091.png)
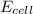 = electrode potential of the cell = ?
= electrode potential of the cell = ?![[H^{+}]=1.39M](/tpl/images/0627/3491/e38d3.png)
![[Sn^{2+}]=9.35\times 10^{-4}M](/tpl/images/0627/3491/dda0a.png)