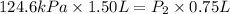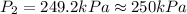Chemistry, 25.04.2020 23:17 WonTonBagel
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experience to have a volume of 0.75 L?
A. 130 kPa
B. 97 kPa
C. 250 kPa
D. 62 kPa

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2


Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experi...
Questions




















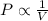
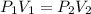
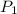 = initial pressure = 124.6 kPa
= initial pressure = 124.6 kPa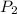 = final pressure = ?
= final pressure = ?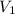 = initial volume = 1.50 L
= initial volume = 1.50 L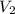 = final volume = 0.75 L
= final volume = 0.75 L